Update: New drug combination for the second treatment of some HR-positive advanced breast cancers
The FDA approved Truqap plus Faslodex for the treatment of metastatic hormone receptor-positive (HR-positive), HER2-negative breast cancer that returned or worsened after treatment with hormone therapy. The approval is for treating cancers in people whose tumors had a mutation in one of three genes. For people with one of these mutations, Truqap improved the time until their cancer came back or got worse. (Posted 2/9/24)
Este artículo está disponible en español.

RELEVANCE
Most relevant for: People with hormone receptor (HR)-positive or HER2-negative breast cancer that has spread outside the breast tissue..
It may also be relevant for:
- people with breast cancer
- men with breast cancer
- people with Her2-positive cancer
- people with metastatic or advanced cancer


Relevance: Medium-High


Research Timeline: Post Approval
What is this update about?
Truqap (capivasertib) plus Faslodex (fulvestrant) can now be used for some people with advanced breast cancer. Faslodex is a type of hormone therapy that is given as an injection. Truqap is a pill taken by mouth; it works by stopping cancer cells from multiplying.
The approved this combination as a second treatment in people with locally advanced or HR-positive, breast cancer in the following situations:
- People whose breast cancer recurred and worsened after receiving hormone therapy.
- People whose breast cancer recurred while they were on their first treatment or within 12 months of completing it.
Truqap is approved only for people with a tumor that has a mutation in one of three genes: PIK3CA, ATK1 or . The Foundation One CDx tumor test was also approved by the for use in identifying people who are most likely to benefit from this new combination treatment.
Study findings
The approval was based on the CAPItello-291 study, which compared Truqap plus Faslodex to Faslodex alone. Just over 700 people with locally advanced or HR-positive, breast cancer participated in this trial. One group received Truqap plus Faslodex, while the other group received Faslodex alone.
Researchers looked at the time until cancer returned in both groups.
Among all participants:
- Cancer did not return for about 7 months in the group that received Truqap plus Faslodex compared to about 4 months in the group that received Faslodex alone.
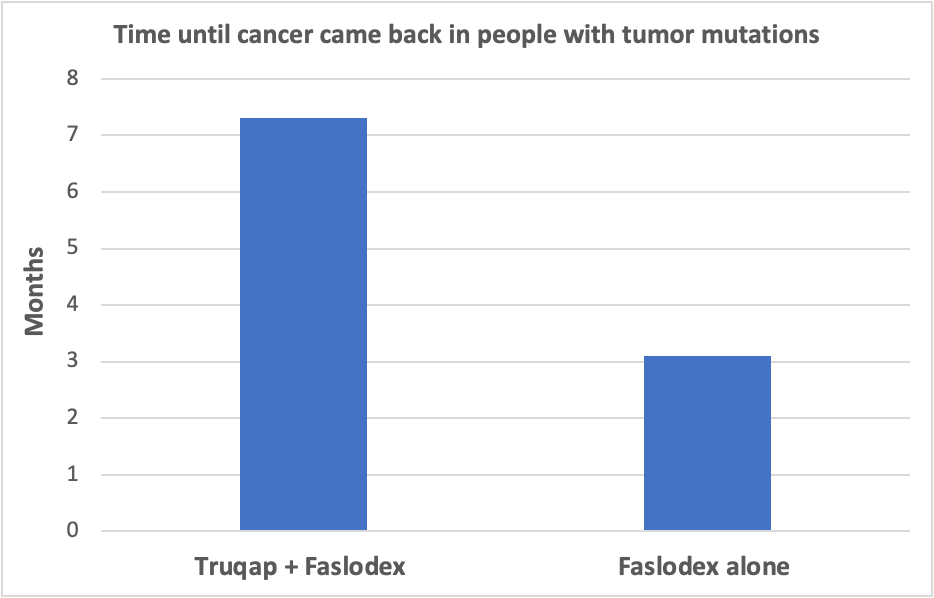
When researchers looked at the subset of participants with a tumor mutation in PIK3CA, ATK1 or PTEN:
- Cancer did not return for just over 7 months in the group that received Truqap plus Faslodex compared to 3 months in the group that received Faslodex alone.
At 18 months, overall survival was higher in the group that received Truqap plus Faslodex compared to the group that received Faslodex alone.
Truqap side effects
Truqap side effects included:
- Rash
- Diarrhea
- High blood sugar
- Hyperglycemia (need insulin and hospitalization)
Among patients who received Truqap, rash and diarrhea were the most common side effects reported. Hyperglycemia (high blood sugar) occurred in 18 percent of these patients. Grade 3 hyperglycemia (need insulin and hospitalization) or Grade 4 (life-threatening) occurred in 2.8 percent of patients.
Fasting blood glucose and hemoglobin A1C levels should be checked for normal levels before taking Truqap. People taking Truqap who have a medical history of diabetes should be monitored more frequently.
What does this mean for me?
If you have or locally advanced or breast cancer that recurs after your first treatment, ask your healthcare provider about testing your tumor to see if you may be eligible for Truqap plus Faslodex or another targeted treatment.
The new drug combination may increase the time until your cancer comes back, especially if your tumor has a mutation in a PIK3CA, ATK1 or gene. It may also help to maintain your quality of life and delay time until chemotherapy is needed.
Reference
approves capivasertib with fulvestrant for breast cancer. news release. November 16, 2023.
Disclosure: FORCE receives funding from industry sponsors, including companies that manufacture cancer drugs, tests and devices. All XRAYS articles are written independently of any sponsor and are reviewed by members of our Scientific Advisory Board prior to publication to assure scientific integrity.
Share your thoughts on this XRAY review by taking our brief survey.
posted 2/9/24
Tumor testing can help determine whether a person with breast cancer would benefit from a or .
| Subtype | Type of Drug | |
|---|---|---|
| ATK1, PIK3CA or mutation | , | |
| ESR1 mutation | , | SERD (a type of hormone therapy) |
| protein | Triple-negative breast cancer () |
|
| MSI-H, , TMB-H | Any | |
| protein | , HER2-low or HER2-ultralow, some |
Anti-HER2 therapy |
| NTREK, RET or NRG1 gene fusions | Any subtype |
*MSI-H or microsatellite instability-High, or , and TMB-H or tumor mutational burden-High are results of three different but related tumor tests. These results indicate that there may be an underlying problem with a type of repair called mismatch repair.
Updated: 07/01/2025
The following studies look at treatment for people with HER2-positive breast cancer:
- NCT05458674: Tucatinib+Trastuzumab+Eribulin in HER2+ MBC. This study evaluates the safety and efficacy of combining the drugs tucatinib, trastuzumab and eribulin in patients with unresectable HER2-positive breast cancer after prior treatment with a taxane, trastuzumab and T-DM1.
- NCT06100874: A Single-arm Phase II Trial of SAcituzumab Govitecan and Trastuzumab for HER2+ Breast Cancer After Trastuzumab dEruxtEcaN (SATEEN). This study evaluates the safety and effectiveness of sacituzumab govitecan with trastuzumab (Herceptin, Herceptin Hylecta or trastuzumab ) in HER2+ breast cancer.
- NCT06435429: A Study Comparing the Efficacy and Safety of Zanidatamab to Trastuzumab, Each in Combination With Physician's Choice Chemotherapy, for the Treatment of Participants With HER2-positive Breast Cancer. This study evaluates the safety and effectiveness of zanidatamab combined with chemotherapy compared to trastuzumab (Herceptin) combined with chemotherapy to treat participants with HER2-positive breast cancer who have progressed on or are intolerant to previous T-DXd treatment.
- NCT05378464: Adoptive T Cell Therapy Following HER2-Pulsed Dendritic Cell Vaccine & Pepinemab /Trastuzumab in Patients w/ HER2+ Breast Cancer. This study tests the safety of Adoptive T-Cell therapy following the Dendritic Cell (DC1) vaccine given in combination with pepinemab added to standard-of-care therapy trastuzumab for people with breast cancer.
- NCT05894239: A Study to Evaluate the Efficacy and Safety of Inavolisib in Combination With Phesgo Versus in Combination With Phesgo in Participants With PIK3CA-Mutated Locally Advanced or Breast Cancer. This study looks at the safety and effectiveness of inavolisib combined with Phesgo (pertuzumab, trastuzumab and rHuPH20 injection) compared with a combined with Phesgo for after induction therapy for participants with previously untreated advanced breast cancer.
Other clinical trials for people with breast cancer can be found here.
Updated: 05/07/2025
The following organizations offer peer support services for people with or at high risk for breast cancer:
- FORCE peer support:
- Our Message Boards allow people to connect with others who share their situation. Once you register, you can post on the Diagnosed With Cancer board to connect with other people who have been diagnosed.
- Our Peer Navigation Program will match you with a volunteer who shares your mutation and situation.
- Connect online with our Private Facebook Group.
- Join our virtual and in-person support meetings.
- Other organizations that offer breast cancer support:
Updated: 05/07/2024