PUBLISHED: 12th July 2011

There is a need for more treatment options for hereditary cancer, and the need is dire for women with metastatic hereditary breast cancer who make up a small subset of the larger breast cancer population. To better understand advanced hereditary breast cancer and to advocate for more research and better treatment options, FORCE conducted a survey for women who have received a diagnosis of metastatic hereditary breast cancer. This survey is still open to women who:
- have a BRCA mutation or breast cancer runs in their family
- have been diagnosed with stage IV (metastatic) breast cancer
As part of the hereditary cancer research updates session at our conference I presented findings from this ongoing survey. Below is a summary of my presentation of preliminary survey findings. We are actively working with researchers to further evaluate the results and help determine the next steps for research.
Conference presentation excerpts
We first introduced the importance of our community setting a hereditary cancer research agenda at our 2008 annual conference. One brave participant came up to the floor microphone during the hereditary cancer research panel and said the following:

“My name is Caryn Rosenberg and I’m 47 years old. Please pardon my nervousness but I feel like I am literally begging for my life. I am BRCA1 positive when I was originally diagnosed it was stage II…cured.
And I am now, as of last year, in stage 4. When I tell people that, their eyes glaze over….’Next. We can’t help you.’
I am a daughter of a holocaust survivor so survivorship comes to me in my DNA. And I know that there are thousands of women like me that are looking for treatment in our stage 4 that will keep us alive, chronic if you will, until a cure can be found.
What I’m finding about most of the medical community is that once they diagnosis stage 4 they want to step on by you and get to the next person that they can help. So…It’s not so much a question as much as an invitation or kind of a demand. I’m out here on the skinny branches with a form of cancer that doesn’t have much research on it, and I am calling to you, I’m begging you to do what you can so that I can live a longer life so I can make a difference. I don’t know what that takes. I’m willing to help however I can.”
Caryn’s eloquent comments were made three years ago. And yet, despite pretty early promising results for some breast cancer drugs, instead of more options we have fewer! The FDA is trying to take away the breast cancer indication for Avastin. We are not much further along than we were three years ago on PARP inhibitor research with no FDA approval yet which means the drug is unavailable outside of the research setting. There is no “compassionate use” availability of any PARP inhibitor. Sadly, I know that Caryn never had the opportunity to benefit from PARP inhibitors because she did not qualify for any of the studies. This MUST change! Caryn died in December 2010--last year. Caryn, is no longer here to speak out for our community. So we must speak out for her and others. It is for Caryn and other members of our community battling stage IV disease that we developed this survey to give them a voice and hope.
Our metastatic community often feels like a forgotten group. Although PARP inhibitor research has been promising we still do not have any large registration clinical trials that we lead us to FDA approval soon. So our “met-her” community is literally out on the skinny branches. We conducted the following survey to learn more about our metastatic community and to include their input in our research priorities. Among the interesting findings:
We asked people: “What was your initial stage of diagnosis?”
Shockingly 21% were first diagnosed with stage 0 or I before their recurrence, which are very early stages. At a point when some groups are calling for LESS treatment for early stage breast cancer (particularly DCIS) this is an alarming number.
And 24% were first diagnosed at stage IV, so early detection is not picking up all of our cancers. Also surprising is the fact that more people who were ER+ than triple negative and more women who are BRCA 2 than BRCA 1 indicated that they were first diagnosed at stage IV.
The age range for diagnosis with metastatic breast cancer was 28 – 68 with a mean age of 47.
For each medication that you have taken since being diagnosed with metastatic breast cancer, please indicate whether or not your oncologist feels the cancer responded to the treatment
When we asked about response to specific treatments, carboplatin, a chemotherapy that is widely used with ovarian cancer but not used as frequently with breast cancer had one of the most favorable profiles. Researchers have proposed that carboplatin and other platinum-based chemotherapies might work preferentially for cancers in people with BRCA mutations. Interesting is that Bevacizumab (Avastin) appeared to perform as well as or better than other agents that have FDA approval for metastatic breast cancer.
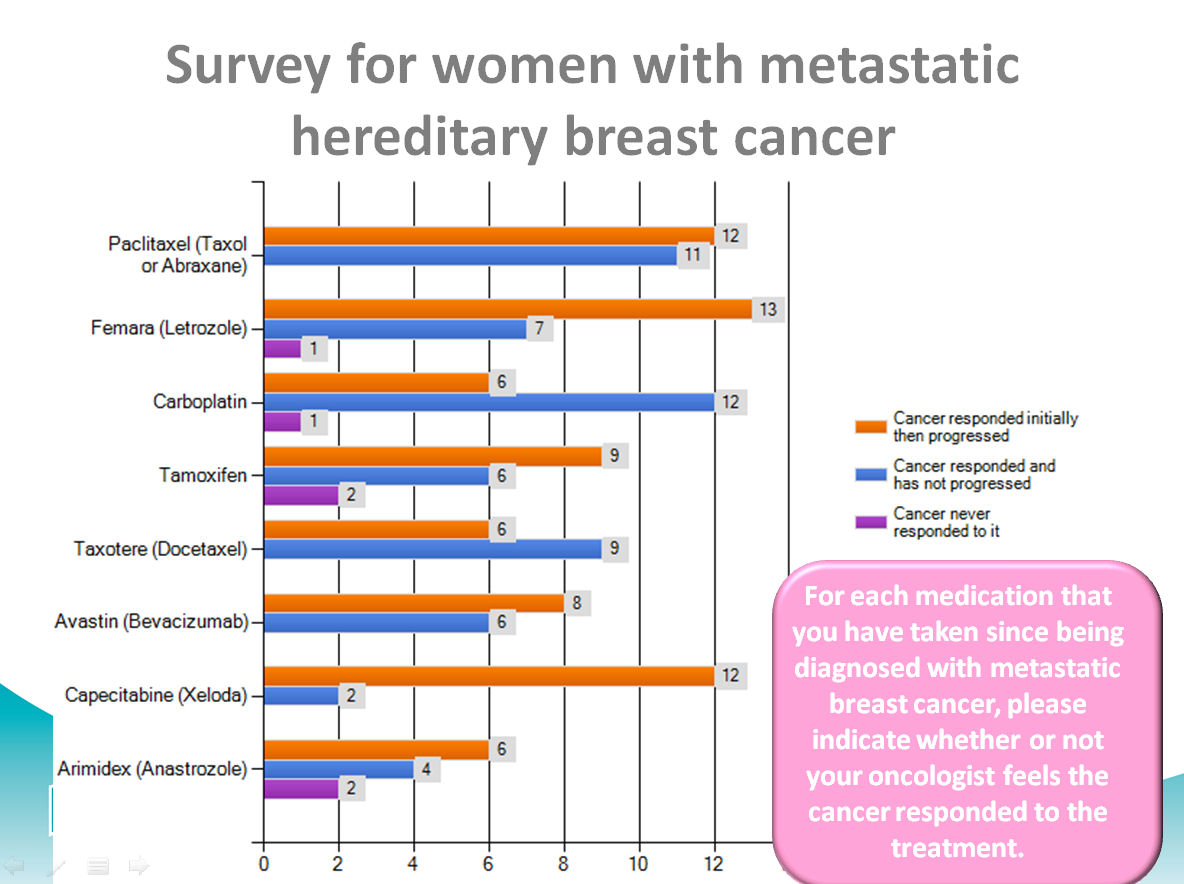
We did not have enough respondents who had taken PARP inhibitors to draw any conclusions about response (iniparib 4 and Olaparib 1).
Is there any other feedback you would like to share with us?
“I would have liked to get on a parp inhibitor trial but wasn't accepted, sadly.”
“I'd like to see more of an emphasis on metastatic hereditary cancer. This is what we're dying of and at much younger ages than women with non-hereditary metastatic breast cancer. I'd also like to see more advocacy for funding directed specifically at metastatic BC.”
“I am the only member in my family who is still alive from this disease.”
Conclusion
Metastatic hereditary breast cancer research may require a different approach than sporadic and these patients may benefit from specific treatments that are different. We need to prioritize making new and promising agents available to this community! IF PARP inhibitors work, we need to figure out a way to get bigger clinical trials, FDA approval, and while we wait, compassionate use.
POSTED IN: Research , Cancer Treatment
TAGS: Breast Cancer , Hereditary Cancer Research , BRCA1 , Metastatic Breast Cancer
5 Comments
July 12, 2011
I wish I could have got on the Parp inhibitor trial but sadly I was refused. My counsillor and others have said it will be licensed soon and I'm very much looking forward to this as I have metastic breast cancer and hopefully the parps will help me live an extra 10 or 20 years.I really wish there was a BRCA support group in the UK.
Elaine Hardy
Reply
July 12, 2011
I was very happy to do the metastic cancer survey and wish the UK had a BRCA support group.
Elaine Hardy
Reply
July 14, 2011
Elaine, please be sure to share this survey with anyone else that you know in the same situation.
Sue Friedman
Reply
August 11, 2011
I think of Caryn almost every day. God, how I wish she were still with us.
August 11, 2011
She left her beautiful, powerful, courageous, and indelible mark on all of us who had the honor of knowing her.
facingourrisk
Reply