PUBLISHED: 12th October 2021
by Susan Jenks
Note: This blog was originally published by the National Cancer Institute
Researchers in gynecologic oncology have begun testing a promising surgery for premenopausal women at high genetic risk for ovarian cancer that avoids early menopause and may prevent these malignancies from developing.
Studies have shown that most ovarian cancers actually begin to grow from cancer cells that developed in the fallopian tubes. This trial is testing if removing the fallopian tubes before menopause prevents ovarian cancer.
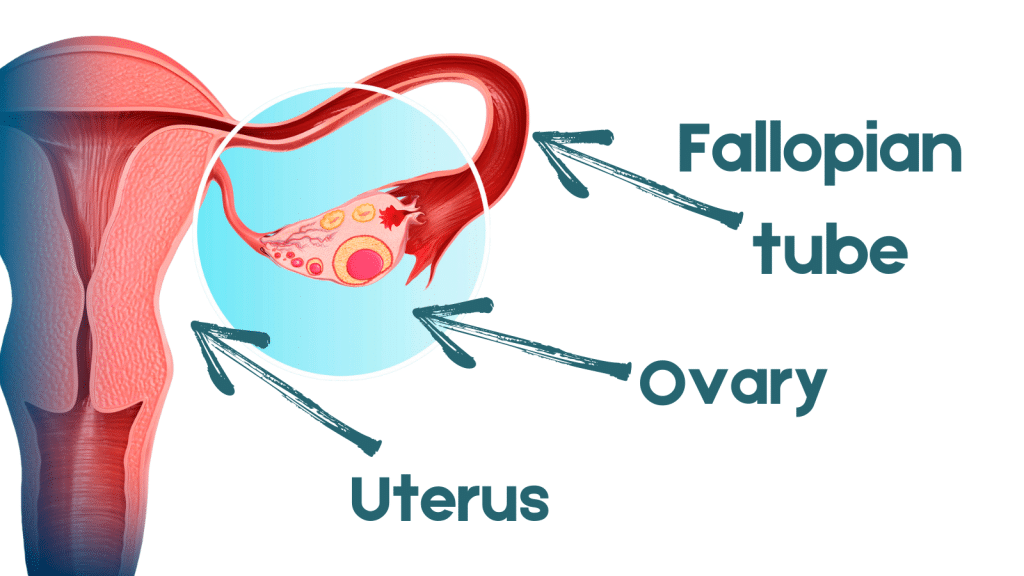
In a large-scale clinical trial sponsored by the National Cancer Institute, researchers hope to determine whether this approach is equivalent to the prevailing surgical standard of care for women testing positive for mutations in their BRCA1 genes: oophorectomy, or the removal of both ovaries and the fallopian tubes, before age 40 years (see image of female reproductive organs).
At-risk women in the trial can self-select for the removal of their fallopian tubes only while delaying further surgery to take out the ovaries, for up to 5 years, at an age closer to natural menopause and the ovarian function wanes.
Although the standard, more invasive procedure “is great for stopping these cancers, it’s not a great option for a younger woman’s quality of life,” said Joan Walker, M.D., the study’s principal investigator and gynecologic oncologist at the Stephenson Cancer Center in Oklahoma City. Surgery to remove both ovaries and fallopian tubes is estimated to reduce risk of ovarian cancer by 98%.
Surgically removing the ovaries induces a drop in estrogen levels similar to what occurs in menopause. Not only are the hot flashes associated with early menopause likely to occur, there is also a possible increase in sexual dysfunction and health risks, such as osteoporosis. However, the “tubes out first, ovaries later,” approach may offer high-risk women a less disruptive lifestyle choice, Dr. Walker said, while cutting off many of these cancers at their suspected source.
“The problem is that the fallopian tubes produce cells that push their way into the ovaries through the fimbria,” she said, referring to the distal fringes at the tubes’ edges. “By removing the tubes, we should be able to prevent ovarian cancer,” she said. Growing scientific evidence now suggests many of these cancers arise from this site and not the ovaries, as once thought.
Anyone Free of Ovarian Disease Can Enroll
The study, called SOROCk, which stands for Salpingo-Oophorectomy to Reduce the risk of Ovarian Cancer (NCT04251052), is sponsored by NRG Oncology, a research organization that is part of the NCI Community Oncology Research Program (NCORP) national network, which brings cancer clinical trials and care delivery studies to people in their communities.
Investigators hope to enroll 2,262 women between the ages of 35 and 50 years old, in the proof-of-principle study. Women will choose either salpingectomy (removal of the fallopian tubes only) to preserve ovarian function, or traditional bilateral salpingo-oophorectomy. Some 190 clinical sites globally are expected to participate in the trial, which will follow the women over 20 years for signs of cancer. Besides testing positive for a BRCA1 mutation, participants must have fallopian tubes.
In addition to answering questions about the ability of the surgery to prevent ovarian cancer, participants are having blood drawn and stored, for future studies of biomarkers to further understand cancer risk.
So far, more than 65 women have enrolled in the trial, Dr. Walker said, but many women in the patient-advocacy community strongly support it and oncologists, like Dr. Walker, have begun reaching out to primary care physicians, genetic counselors and others, to alert them to the prevention study’s recruitment needs.
“We invite any woman who carries these mutations and is free of ovarian disease to consider being part of this study.” she said, even women who’ve had mastectomies to lower their breast cancer risk, which also rises with BRCA1 mutations. Women who have discovered they carry the BRCA1 mutation after being diagnosed with breast cancer at an early age are eligible for the study, as that is often the only way women know they are carriers.
Although inherited mutations in the BRCA2 gene carry an elevated risk, as well, for both ovarian and breast cancers, investigators decided against including this additional risk group for statistical reasons, she said. Not only do fewer women develop ovarian cancer who carry this genetic mutation, disease onset tends to come later, usually 10 to 15 years after menopause.
Unlike breast cancer, where effective screening strategies provide guidance for women facing a higher-than-average cancer risk, none yet exists for the early detection of ovarian cancer.
About 80% of ovarian cancers are diagnosed in advanced stages when treatment options are few and 5-year relative survival declines. Biomarkers also remain ill-defined, experts say, with the CA-125 antigen considered the best available, yet of limited value, except for tracking disease recurrence.
Genetic Testing and Research Focus on How Best to Reduce Cancer Risk
In Canada, public policy mandates that women who undergo hysterectomies have their fallopian tubes taken out, even if no cancer is present. But the impact on ovarian cancer incidence in the country is expected to take years to determine and these “opportunistic surgeries,” as they are known, affect average-risk women, not those at high-risk for the disease.

Women on the SOROCk trial have a molecular profile that renders them far more vulnerable to the development of ovarian cancer than the norm. The average lifetime risk for ovarian cancer is about 1.2% compared with 40% by age 80 in women born with mutated BRCA1 genes.
Uncertainty about how best to reduce that risk, however, causes understandable frustration in women who know they carry these deleterious genes and may pass them on to their children and grandchildren, and which may already affect other close relatives.
“We’re really invested in this type of research—there’s a lot of interest,” said Sue Friedman, the executive director and founder of Facing Our Risk of Cancer Empowered (FORCE)(link is external), which advocates for individuals with any type of hereditary cancer. “Most women don’t make the connection that they’re part of the population that can help us get the answers we need for the future.”
So, if genetic testing shows they’re eligible, women “need to show up,” for this study, according to Friedman, a 20-year survivor of hereditary breast cancer. Otherwise, she worries, the information they need to make informed medical choices for themselves and their families still will be lacking, perhaps for many years.
“This is an important moment in time because it took upwards of 8 years to design the trial and required a big financial investment from the NCI,” Friedman said. And, while long-term data show removing the ovaries and fallopian tubes will help you live longer, women need similar data to prove this alternative approach may be a better option, she said.
For more information about SOROCk, visit or call the NCI Cancer Information Service, 1-800-4-CANCER (1-800-422-6237) or visit the SOROCk study listing on the FORCE website.
POSTED IN: Research , Risk Management
TAGS: Ovarian Cancer , BRCA1 , Previvor , Prophylactic Surgery , Screening And Prevention