Pancreatic Cancer Screening Guidelines
What is pancreatic cancer screening?
Screening for pancreatic cancer uses tests to try to catch cancer in its early stages, when it is most treatable. The earlier that cancer is found, the better a person’s chance of surviving it.
Types of pancreatic cancer screening
There are two main types of screening used to try to find pancreatic cancer at an earlier and treatable .
- Contrast-enhanced magnetic resonance cholangiopancreatography (MRCP) is a special type of imaging that looks closely at the pancreas, liver, gallbladder, bile duct and pancreatic duct to find abnormalities such as cancer. People undergoing MRCP must fast for four hours before the procedure. An injection of contrast agent—called gadolinium—is given before the test in order to help radiologists to find abnormalities more easily.
- Endoscopic (EUS) involves passing a tiny scope with an attached probe down the esophagus to the stomach. This allows doctors to look closely at the pancreas. EUS is performed as an outpatient procedure under anesthesia.
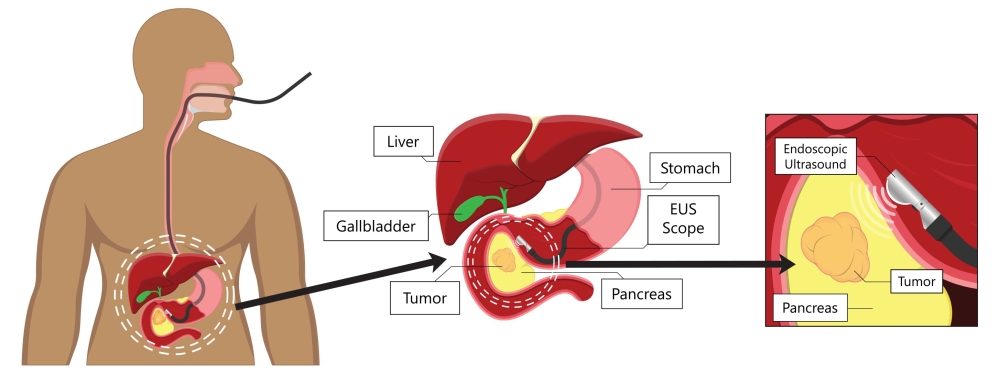
Endoscopic procedure to look for pancreatic tumors
Expert guidelines
Experts do not recommend screening healthy people at average risk for pancreatic cancer. In people at high risk for pancreatic cancer, screening is only recommended for certain people. There are two professional groups with guidelines for pancreatic cancer screening for high-risk people that differ in their recommendations, NCCN and ASGE.
National Comprehensive Cancer Network (NCCN) guidelines
NCCN recommends that people undergoing pancreatic screening have the procedure at a facility with experience screening people at high risk for pancreatic cancer. Before undergoing screening, people should have a conversation with their doctor about the potential benefits, risks, costs and limitations of screening.
Guidelines for people with a mutation, regardless of family history of pancreatic cancer
NCCN recommends that people with inherited mutations in the following genes (with or without a family history of cancer) consider annual pancreatic cancer screening with MRCP or EUS.
- (): Consider pancreatic cancer screening by MRCP or EUS beginning at age 30-35 or 10 years younger than the earliest pancreatic cancer in the family.
- : Consider pancreatic cancer screening beginning at age 40 or 10 years earlier than the earliest pancreatic cancer diagnosis in the family.
- and : Consider pancreatic cancer screening beginning at age 50 or 10 years earlier than the earliest pancreatic cancer diagnosis in the family.
Guidelines for people with a mutation and a family history of pancreatic cancer
NCCN guidelines recommend that people with an in one of the following genes and a family history of pancreatic cancer consider annual pancreatic cancer screening with MRCP or EUS beginning at age 50 or 10 years earlier than the earliest pancreatic cancer diagnosis in the family:
, , , , , , .
Guidelines for people with a mutation linked to hereditary pancreatitis and a personal history of pancreatitis
People with a mutation in PRSS1 or SPNK1 and evidence of pancreatitis should consider annual screening beginning 20 years after the onset of pancreatitis symptoms or by age 40 (whichever is earlier).
|
Gene |
Family History |
Beginning Age |
Recommendation |
|
No family history of pancreatic cancer required |
30-35 (or younger based on the earliest pancreatic cancer in the family) |
Consider pancreatic cancer screening by MRCP or EUS every year. |
|
|
No family history of pancreatic cancer required |
40 (or younger based on the earliest pancreatic cancer in the family) |
Consider pancreatic cancer screening by MRCP or EUS every year. |
|
|
No family history of pancreatic cancer required |
50 (or younger based on the earliest pancreatic cancer in the family) |
Consider pancreatic cancer screening by MRCP or EUS every year. |
|
|
Requires at least one first- or with pancreatic cancer. |
50 (or younger based on the earliest pancreatic cancer in the family) |
Consider pancreatic cancer screening by MRCP or EUS every year. |
|
|
Source: NCCN Guidelines: Genetic/Familial High-Risk Assessment: Breast, Ovarian, Pancreatic, , v. 2 2026. |
|||
American Society for Gastrointestinal Endoscopy (ASGE) guidelines for people with a or mutation
The ASGE released guidelines on pancreatic cancer screening for people with a and mutation. These guidelines are different from NCCN guidelines for people with and mutations. ASGE recommends:
- All people with a or mutation regardless of family history of pancreatic cancer, should undergo annual screening for pancreatic cancer with MRI/MRCP or EUS beginning at age 50 (or 10 years earlier than the earliest pancreatic cancer in the family).
Pancreatic Cancer Early Detection Consortium
The PRECEDE Consortium is a collaboration of experts working to improve detection and prevention of hereditary pancreatic cancer.
NCCN Patient Guidelines for Pancreatic Cancer
The National Comprehensive Cancer Network has patient guidelines to help people diagnosed with pancreatic cancer make informed decisions.
Let's Win! Pancreatic Cancer
Let's Win is a go-to guide with easy-to-understand, actionable content focused on the needs of the patient and caregiver.
Vaccine for People at High Risk for Pancreatic Cancer
Clinicaltrials.gov identifier: NCT05013216
Screening Study for Pancreatic Cancer in People with Inherited Mutations
Clinicaltrials.gov identifier: NCT05058846
Pancreatic Cancer Screening Study (CAPS5)
Clinicaltrials.gov identifier: NCT02000089
Pancreatic Cancer Early Detection for People at High Risk
Clinicaltrials.gov identifier: NCT04970056
Blood Markers of Early Pancreas Cancer
Clinicaltrials.gov identifier: NCT03568630
Pancreatic Cancer Screening Study for High Risk People
Clinicaltrials.gov identifier: NCT03250078