The DETECT Study: Detecting Endometrial Cancer in Tampons
Prevention
Early detection of endometrial cancer in women undergoing hysterectomy surgery at the University of Alabama
Clinicaltrials.gov identifier:NCT03538665
Study Contact Information:
For additional information, please contact:
Rebecca C Arend, MD by phone at (917) 532-5370 or Email: rarend@uabmc.edu
Megan A Clarke-Corso, Ph.D. by phone at (240) 276-7823 or Email: megan.clarke@nih.gov
About the Study
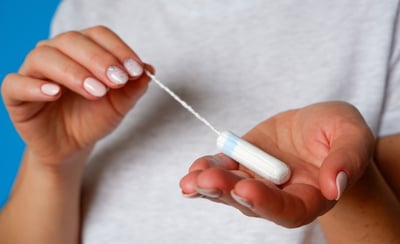
Women over 45 years of age who are scheduled for a hysterectomy for endometrial cancer, endometrial cancer precursors, or benign conditions at the University of Alabama’s Division of Gynecological Oncology will use vaginal tampons to test whether samples collected on the tampon can detect endometrial cancer.
This Study is Open To:
Women, 45 years and older, who meet the following criteria:
- Will undergo hysterectomy (surgery to remove the uterus) for endometrial cancer, precursors or atypical hyperplasia (early changes that can lead to endometrial cancer), or benign condition including fibroids, , endometrial hyperplasia, adenomyosis, and normal endometrium at the University of Alabama Birmingham Division of Gynecologic Oncology
- Have the ability to understand the study and give informed consent
This Study is NOT Open To:
- Women under 45 years old
- Pregnant women
What the Study Involves
Prior to surgery, participants will be given a 10-question survey to see if they find tampon sampling acceptable.
They will then put a tampon in their vagina at least 30 minutes before surgery. Their tampons will be collected during the surgery. A small piece of uterine tissue will be removed during surgery. Researchers will study both tampon and tissue samples.
Researchers will follow the participant’s medical records for up to 3 years.
Lead Researcher
Rebecca C. Arend, MD,University of Alabama at Birmingham
Study Contact Information:
For additional information, please contact:
Rebecca C Arend, MD by phone at (917) 532-5370 or Email: rarend@uabmc.edu
Megan A Clarke-Corso, Ph.D. by phone at (240) 276-7823 or Email: megan.clarke@nih.gov
Locations:
Alabama
City: Birmingham RECRUITING
Facility: University of Alabama at Birmingham
Contact Info:
rarend@uabmc.edu
205-934-5370
Prevention
Early detection of endometrial cancer in women undergoing hysterectomy surgery at the University of Alabama
Clinicaltrials.gov identifier:NCT03538665
Study Contact Information:
For additional information, please contact:
Rebecca C Arend, MD by phone at (917) 532-5370 or Email: rarend@uabmc.edu
Megan A Clarke-Corso, Ph.D. by phone at (240) 276-7823 or Email: megan.clarke@nih.gov