Upright MRI for Prostate Cancer Screening
Prevention
Screening for prostate cancer using upright MRI
Clinicaltrials.gov identifier:NCT03474913
Study Contact Information:
For additional information, please contact:
Study Director: Dara Lundon (212) 241-0751 Dara.Lundon@mountsinai.org
About the Study
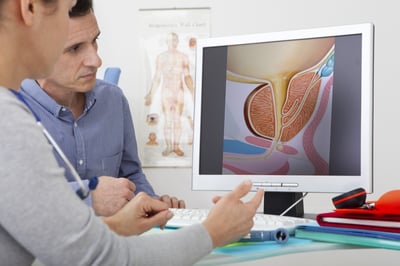
This study will compare the effectiveness of an upright magnetic resonance imaging () compared to specific antigen () and current imaging for cancer screening.
This Study is Open To:
Men 18 years and older who:
- Are at risk of cancer and have been told to have a MRI
- Can undergo all parts of the study including guided biopsy
- Can tolerate general or spinal anesthesia
- Ability to understand and the willingness to sign a written informed consent and to comply with the protocol.
This Study is NOT Open To:
Those who do not meet the criteria defined above and individuals who:
- Have been treated with 5-alpha-reductase inhibitors (marketed as Proscar, Propecia, or Avoadart) within 6 months before beginning the study
- Have had a previous biopsy, surgery, or treatment for cancer
- Have a urinary tract infection or history of acute prostatitis, inflammation of the , within the last 3 months
- Cannot have an
What the Study Involves
Participants will have a standard of care as well as an upright, seated . After each , participants will be asked to fill out questionnaires to assess their comfort level during the scans. If something suspicious is found on either scan, participants may have an guided biopsy of the , a way to sample and test tissue for cancer. Researchers will check in with the participants every 6 months for 5 years.
Lead Researcher
Ash Tewari: Icahn School of Medicine at Mount Sinai
Study Contact Information:
For additional information, please contact:
Study Director: Dara Lundon (212) 241-0751 Dara.Lundon@mountsinai.org
Locations:
New York
City: New York RECRUITING
Facility: Icahn School of Medicine at Mount Sinai
Contact Info:
Ash Tewari, MD
Prevention
Screening for prostate cancer using upright MRI
Clinicaltrials.gov identifier:NCT03474913
Study Contact Information:
For additional information, please contact:
Study Director: Dara Lundon (212) 241-0751 Dara.Lundon@mountsinai.org